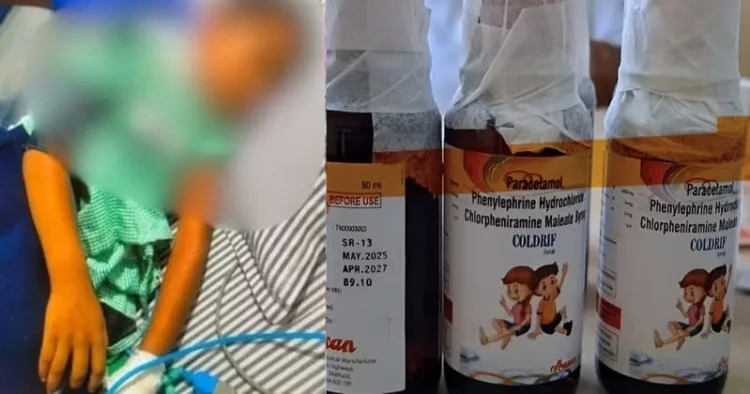
In a deeply tragic incident, eleven children under the age of five have died in Madhya Pradesh’s Chhindwara district after consuming a contaminated cough syrup, Coldrif. The syrup, prescribed by a local paediatrician, was later found to be laced with diethylene glycol (DEG), a toxic industrial chemical used in antifreeze and brake fluid, known to cause acute kidney failure and death when ingested.
The doctor, Praveen Soni, posted at the Civil Hospital in Parasia, was arrested following an investigation. He reportedly prescribed the syrup during his private practice to infants who had been brought to him with mild symptoms of cold, cough, and fever. Within days, the children began showing signs of acute kidney injury, including reduced urine output and elevated creatinine and urea levels. Ten of them died during treatment at Nagpur’s Government Medical College and Hospital, and another child’s biopsy confirmed acute tubular injury. Five more children remain hospitalised.
Early October 5 morning, Madhya Pradesh Police registered an FIR at the Parasia police station based on a complaint from Dr Ankit Sahlam, the Block Medical Officer at the Community Health Centre. The FIR, lodged at 2:05 am, names Dr Praveen Soni, the director of Sresan Pharmaceuticals, the Tamil Nadu-based manufacturer of Coldrif, and other responsible parties involved in the drug’s production and distribution. The case has been registered under Sections 105 and 276 of the Bharatiya Nyaya Sanhita and Section 27(a) of the Drugs and Cosmetics Act, 1940.
Lab tests confirmed the contamination. A report from the Director of Drugs Control, Tamil Nadu, dated October 2, 2025, found that the syrup (Batch No. SR-13, manufactured in May 2025 and expiring in April 2027) contained 48.6% diethylene glycol. A separate test by the Government Drug Testing Laboratory in Bhopal found 46.28% of the same toxic compound. Both labs declared the samples adulterated and harmful to health.
In response, the Madhya Pradesh government swiftly banned the sale of Coldrif and issued urgent orders to seize all available stock. The Food and Drug Administration also instructed drug inspectors to collect samples from other batches for testing, and extended the ban to all medicines produced by Sresan Pharmaceuticals.
Health Commissioner Tarun Rathi suspended Dr Soni, stating that he was found conducting private practice in violation of rules, during which he prescribed the harmful syrup. The inquiry revealed that his negligence in examining and diagnosing the infants, coupled with the prescription of tainted medication, led to the children’s deaths. The suspension order said that his actions caused irreparable damage and brought disrepute to the department.
The incident, first reported in late August and concentrated in the villages of Parasia tehsil, has sparked nationwide concern. Similar cases in Rajasthan have prompted a wider investigation. The Central Drugs Standard Control Organisation (CDSCO) has launched inspections of pharmaceutical manufacturing units across six states,Himachal Pradesh, Uttarakhand, Gujarat, Tamil Nadu, Madhya Pradesh, and Maharashtra, particularly focusing on cough syrups, antipyretics, and antibiotics produced in regions where such fatalities have occurred.
As the investigation continues, both state and central authorities are under pressure to tighten drug quality controls and hold those responsible fully accountable for the tragedy.


















Comments