Imagine a battery that can be bent like paper, folded in two and still drive your device without breaking. Now imagine it as so safe that you can touch it without worrying about burns or explosions. It’s no longer just an imagination it is a turned reality in the making in Bengaluru lab.
Researchers at the Centre for Nano and Soft Matter Sciences (CeNS), autonomous research institute of the Department of Science and Technology (DST), Government of India, in collaboration with researchers from the Centre for Nano Science and Engineering (CeNSE) at the Indian Institute of Science (IISc), have made a breakthrough that may revolutionize the way the world perceives batteries.
Their breakthrough is a stretchable aluminium-based battery that might someday replace the lithium-ion batteries we use in everything from smartphones and laptops to electric cars and wearable technology.
Why This Matters: The Trouble with Lithium-Ion
Lithium-ion batteries have powered electronics for years. They store a lot of power into tiny spaces, making them ideal for our high-tech life. They are not without danger, Lithium-ion cells tend to get overheated and in extreme cases it has exploded also. Stories of phones bursting into flames or electric scooters exploding into flames show just how hazardous the batteries can be.
Lithium is not as abundant, it is critical elements and in mining process it also poses serious environmental costs. As the world moves towards electric mobility and cleaner energy, relying on lithium alone creates economic and ecological pressure.
Rather than using lithium the scientists went to aluminium, which is one of the most prevalent metals on the planet. Aluminium has fascinated scientists for years due to its potential to store and discharge energy. But due to the complexity of the chemistry, aluminium batteries had not been feasible for everyday use.
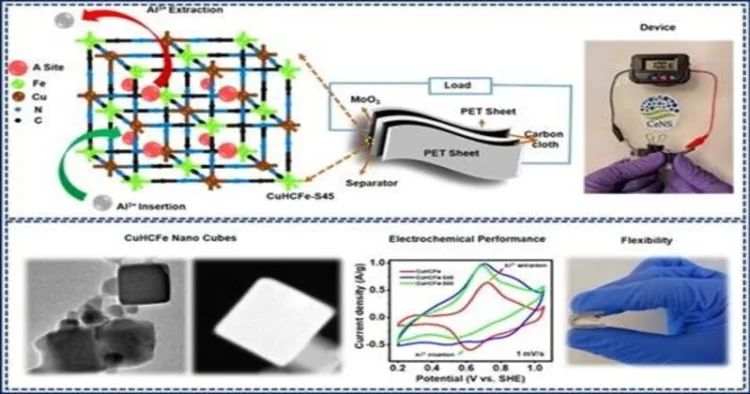
The CeNS and IISc team opted to solve this issue in its fundamental nature by developing the battery materials differently at the microscopic level. This move unlocked the potential of aluminium and created a water-based, aluminium-ion battery that is safer, less expensive and sustainable.
So how did they achieve this? The innovation was in development of the electrodes, the positive and negative component of the battery.
• For the cathode (positive terminal), they used a unique material known as copper hexacyanoferrate (CuHCFe). The material was pre-loaded with aluminium ions, thus allowing the battery to store and discharge energy efficiently.
• For the anode (negative terminal), they employed molybdenum trioxide (MoO₃).
This couple of cathode and anode terminal produced a potent, durable and versatile battery. The uniqueness of the battery is not only limited to store charges but also that it stands up to stress. The battery can be bent, folded and even crushed flat without loss of performance.
Performance and Flexible Future
The new aluminium-ion battery has some encouraging result:
• It retained 96.77% of its strength after 150 charge-discharge cycles. This indicates that the battery is not rapidly depreciated, a significant problem in energy storage systems.
• Scientists showed its versatility by powering an LCD screen continuously, even when the battery was bent at extreme angles.
Functioning of battery was fully up to mark even it is folded in half. This versatility holds a great future where, a phone could be rolled up like a piece of paper or like a clothing that contains onboard power sources for your gadgets. Wearable technology is a multimillion-dollar industry which may become more convenient and comfortable with batteries woven into clothing.
For electric cars the safety of the battery is profitable. Because it is water-based and consists of highly flammable electrolytes and the danger of explosion is highly minimized. This could make the next generation of EV’s safer and cleaner too.
Attaining this milestone was not an easy accomplishment. The scientists used microscopic devices, including electron microscopes and spectroscopic methods to further refine the battery materials. These tools enabled them to observe and gauge how the ions in the electrodes behaved so that everything could harmonize perfectly.
Every step of the process was thoroughly tested to establish efficiency, durability and flexibility. The aggressive process ensures that this technology is not only a lab success but one with prospects for application in the real world.
A Leap in Ion Technology and Sustainable Tomorrow
This innovation represents a major leap in what scientists refer to as multivalent ion battery technology. While lithium-ion batteries shuttle single lithium ions, multivalent batteries such as aluminium-ion supports more than one charge, thus providing more storage capacity. Solving of the age-old difficulties of aluminium chemistry by the Bengaluru team has put India at the forefront of this new field.
Employing aluminium which is a readily available and green resource, implies lower reliance on rare and expensive lithium. A water-based system also adds to safety and greenness. This fits with the global goal of sustainability objectives, where nations are competing towards cleaner, safer and more accessible sources of energy. With the ongoing advancements these batteries might potentially fuel everything from our homes to our vehicles by decreasing environmental threats as well as energy expenses.
India’s Role in the Global Race
The creation of this adaptable aluminum-ion battery is not merely a scientific it is an assertion of India increasing leadership in new-generation energy technology. By investing in the scientific research of institutions such as CeNS and IISc, by supporting them through the Department of Science and Technology, the government is positioning the country at the center of clean energy innovation.
The advantages of this kind of research go far beyond India. Safer, greener batteries are a world necessity. As countries seek to replace lithium, India innovation can potentially be used worldwide.
The findings are exciting, it’s just the start. Scientists now are working on:
•Scalable versions of the battery for bigger uses, such as electric cars and power systems.
•Enhancing the number of charge-discharge cycles it can withstand without losing efficiency.
•Seeking commercial collaborations to take the technology from laboratory to market.
Through further research the stretchable aluminium-ion battery may soon make its way from laboratory tests to daily devices. The tale of this new battery is not merely a scientific story it is one of visions the future of energy. From energizing flexible devices and wearable technology to enabling electric cars to be safer and cleaner the potential is limitless.
Due to the commitment and vision of scientists, India is making it possible to rewrite the rules for energy storage. A paper-thin battery that is safe to the touch could become the foundation for technology tomorrow, demonstrating that innovation can be potent and sustainable.

















Comments