Cancer treatment is often guided by visible signs of tumour size, spread to nearby tissues or the presence of metastasis. For decades, oncologists have known that these indicators, but do not reason to fully explain why two patients with the same cancer stage can respond very differently to treatment. A new study in India now offers a powerful answer through OncoMark, an artificial intelligence framework capable of reading the molecular behaviour of tumours with unprecedented precision. The framework has been developed jointly by the S N Bose National Centre for Basic Sciences, an autonomous institute under the DST and Ashoka University is a major scientific milestone.
New way of seeing cancer
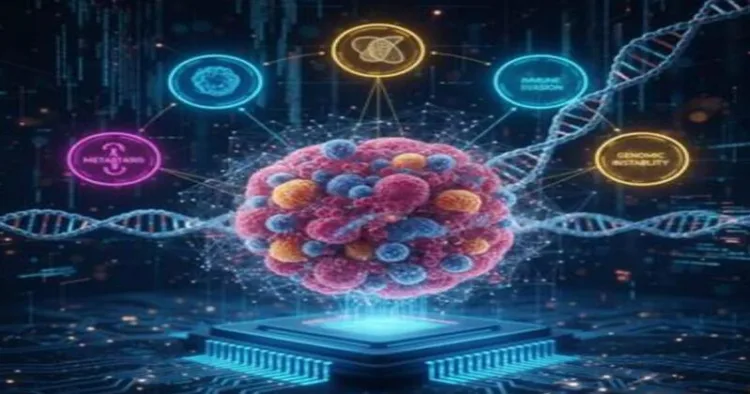
Traditional cancer staging systems, such as TNM, classify tumours on the basis of their size, nodal involvement and metastatic spread. These systems often fail to address a critical issue, such as why cancer progresses. Tumours behave not only as masses of abnormal cells but as biological systems governed by molecular programs. These programs, collectively termed the hallmarks of cancer, drive processes such as sustained growth, immune evasion, resistance to cell death, and invasiveness.
The hallmark concept explains the deeper aspects of malignancy, but translating these molecular insights into clinical tools has been difficult. Existing diagnostic methods do not capture hallmark activity in a reliable, scalable form. This gap has limited the ability of clinicians to personalise treatment according to the internal behaviour of each tumour.
OncoMark attempts to close this gap. Instead of describing how big or widespread a cancer is, it interprets the molecular personality of a tumour using advanced AI models trained on massive single-cell datasets. The result is an analytical tool that connects tumour biology with potential treatment strategies.
Building the largest single cell cancer dataset
To build the framework, the research team, led by Dr Shubhasis Haldar and Dr Debayan Gupta, assembled an extraordinary dataset comprising 3.1 million single cells from 14 cancer types. The AI model learned to recognise the molecular signals associated with key hallmarks by analysing this large spectrum of tumour states.
A significant innovation was the creation of pseudo-biopsies, synthetic samples representing hallmark-driven tumour patterns. These samples helped the AI to understand how hallmark activity evolves as cancer advances. Because real biopsies cannot always capture the full diversity of tumour states, the synthetic dataset offered a more complete map of how biological programs interact inside malignant cells.
Accuracy across real-world cancer
A powerful tool must work reliably beyond laboratory conditions, and OncoMark demonstrates that robustness thoroughly. In internal tests, the system achieved over 99 per cent accuracy and maintained more than 96 per cent accuracy across five independent patient cohorts, including diverse sample types and tumour profiles. The team validated the model using 20,000 real-world patient samples sourced from eight major international datasets. This large-scale validation indicates that OncoMark is not limited to any specific cancer type or data environment.
One of the most important outcomes of this testing was the ability to visualise hallmark activity across cancer stages. For the first time, researchers could clearly observe how molecular hallmarks rise or decline as cancer progresses from early to advanced stages. This will create a more precise picture of tumour progression and allow clinicians and scientists to understand which processes dominate in different stages of the disease.
Clinical Applications: Towards smarter treatment choices
OncoMark’s strength lies not only in its accuracy but also in the potential utility in real world cancer care. Because it identifies which hallmarks are active in a patient’s tumour, it can help doctors match patients to therapies that directly target those processes.
-When a tumour shows strong immune evasion signatures, immunotherapy-focused strategies could be prioritised.
-If genomic instability dominates, then treatments targeting DNA repair mechanisms may prove more effective.
-If the metastatic hallmarks are highly active even at early stages, then aggressive intervention can be planned much sooner.
Such granularity at the molecular level is hardly ever available from conventional staging systems. This makes OncoMark a very promising tool for precision oncology, where decisions are made based on the unique biological characteristics of each patient’s cancer.
Addressing the limits of traditional diagnostics
The recurring challenge in oncology is that two patients can have tumours that appear similar on scans, yet the response to the same treatment can be very different. This variability often proceeds from hidden molecular differences that cannot be picked up by imaging or basic pathology alone.
By offering a direct window on hallmark activity, OncoMark adds a layer of biological interpretation complementary to the existing diagnostic armamentarium. It doesn’t replace TNM staging or biopsy-based pathology, but enhances these with deeper insight into tumour behaviour. The framework identifies cancers that appear less threatening in appearance from the outside yet show molecular signs of internal aggressiveness. This enables clinicians to intervene earlier, especially in cases where the traditional indicators underestimate disease severity.
Landmark for Indian science community
Publication in Communications Biology, which is a part of the Nature Publishing Group, underlines the global relevance and scientific findings of this research. The fact that this state-of-the-art AI framework emerges from Indian institutions further defines India’s leadership position in the domain of computational biology and precision medicine. S N Bose National Centre for Basic Sciences, an autonomous institute under the DST, has been gradually expanding its work in molecular biophysics and computational modelling. Ashoka University’s computational research ecosystem also played a significant role, and together these institutions have positioned India at the forefront of AI-driven cancer research.
The OncoMark framework is not a diagnostic product yet, but it lays the foundation for clinical tools that can combine tumour imaging, pathology and molecular analysis in a unified predictive interface. By decoding how hallmark activity shapes cancer progression, it may help clinicians design more personalised therapies and improve early-stage intervention.
Cancer care is moving away from one-size-fits-all treatment. With frameworks like OncoMark, the future may rely not just on seeing where the tumour is, but understanding what it is truly happening at the molecular level.

















Comments