The Indian scientific ecosystem is making steady progress in finding feasible solutions for energy production that are both sustainable and safe. A breakthrough has been achieved by researchers from Bengaluru at CeNS, an autonomous institute under the Department of Science & Technology (DST). Their breakthrough focuses on improving zinc-ion batteries- a technology increasingly viewed as a safer and more environmentally friendly alternative to the widely used lithium-ion systems.
Lithium-ion batteries have dominated global energy storage due to their high energy density and reliable performance. As safety concerns, fire hazards and environmental impacts during mining and waste disposal have driven scientists to find alternatives. Zinc-ion batteries are emerging as a functional replacement due to the abundance of zinc, which is less hazardous and functions with water-based electrolytes, which reduces the chances of fire and chemical instability. This combination of safety, availability and reduced environmental risk has attracted international research interest.
Despite the huge potential of zinc-ion batteries, one critical bottleneck that has hampered their development is a lack of stable, high-performance cathode materials. A number of oxide-based materials have been tried by researchers around the world, but most have failed to deliver in the long run, especially in successive charge and discharge cycles. High energy storage and lasting stability have thus long been very difficult to attain.
The Bengaluru-based research team led by Dr Ashutosh Kumar Singh rose to the challenge of devising a simple yet effective method to improve a common battery cathode material, vanadium oxide (V2O5). The approach is based on the use of heat and an electric field for restructuring the material in what is described as thermo-electrochemical activation. The objective was to increase the storage and release of energy from the material while retaining stability over repeated charge-discharge cycles.
Activated cathode materials for battery
The central innovation lies in how the team changed the structure of V2O5. The activating treatment creates tiny defects and pathways within the material on purpose. These microscopic changes never weakened it but served to enable the material to function more efficiently by allowing faster, smoother movements of ions when the battery operates. After activation, the material, now zinc-vanadium oxide (Zn-V2O5), offers better interaction with both zinc ions and hydrogen ions in the electrolyte. This improved interaction helps the material maintain its structure while supporting higher levels of energy storage.
One of the practical benefits of this change is that ions are able to move with lower resistance inside the activated material. This improves one of the major limitations of existing active cathode materials used in zinc-ion systems, which often fail to maintain structural integrity upon repeated operation. Not only does the method developed by the team from Bengaluru strengthen this integrity, but it also enhances the material’s performance in general.
Higher energy density and longer battery
Preliminary testing of the new material reveals that the activated Zn-V2O5 significantly enhances the energy density of zinc-ion batteries while supporting a much longer cycle life, a measure of how many thousands of times a given battery can be charged and discharged with minimal loss of efficiency. Cycle life is considered among the most critical parameters to ensure reliability at application levels from consumer electronics to grid-level storage.
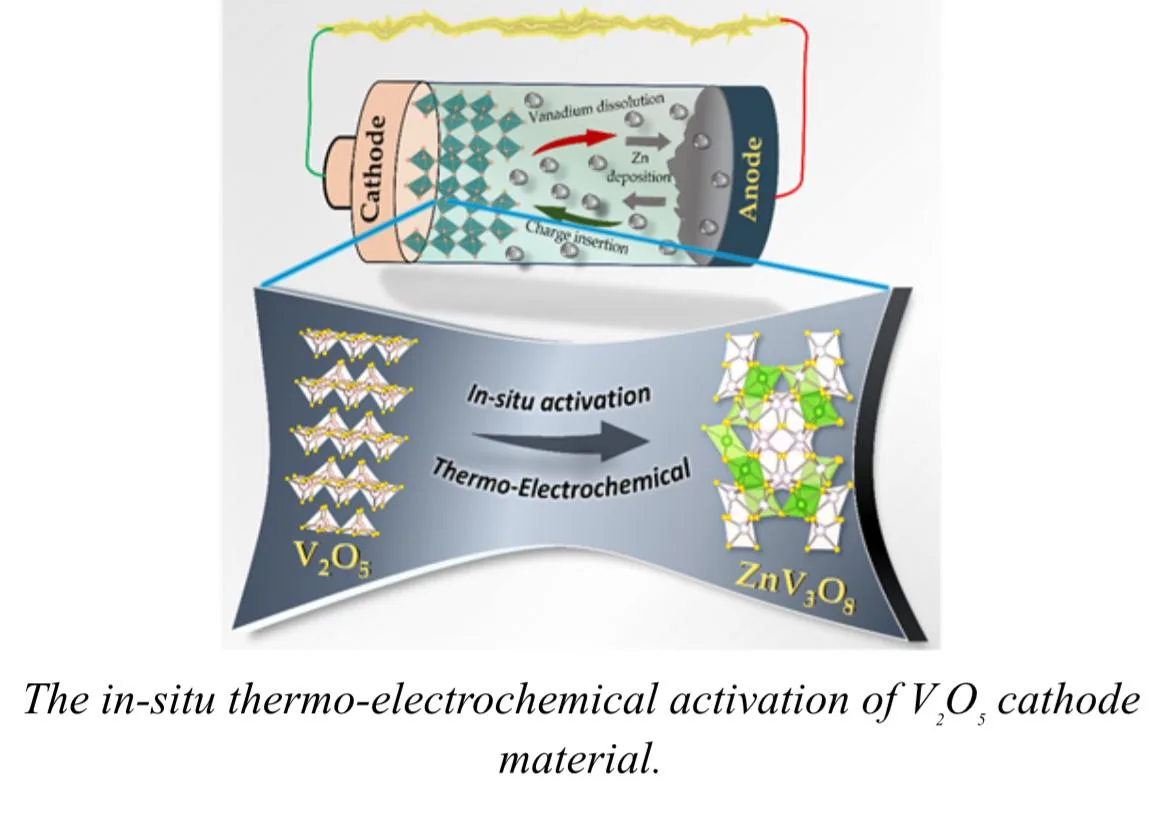
The researchers characterise their method as easy and efficient. This activation process does not deal with complicated chemical modifications or expensive fabrication methods but uses controlled heating in conjunction with an electric field to build a more responsive and stable structure. Due to this simplicity, the technique has provided an accessible option that could be adopted by different laboratories and industries working on zinc-ion technology.
According to co-author Rahuldeb Roy, the team recognised early on how difficult it is to keep cathode materials stable during zinc-ion interaction. Their approach was to design a treatment method that was uncomplicated but still capable of producing significant improvements. The success of their effort now opens up opportunities to apply this activation technique to different types of cathode materials, not just vanadium oxide. That means research groups working on other battery chemistries may benefit from the same strategy, potentially broadening the impact of this discovery.
Step toward sustainable energy storage
Energy storage is a crucial element that empowers modern technologies, from electric mobility to renewable energy systems. As countries around the world are increasingly installing solar and wind power, the need for safer and more reliable battery options is also rising. Technologies in the category of zinc-ion batteries with water-based electrolytes and earth-abundant materials. It also offers a practical path toward sustainable and secure energy infrastructure.
The breakthrough by CeNS strongly aligns with India to push for indigenous research and cleaner energy solutions. Domestic innovations in key sectors such as battery materials reduce dependence on imported technologies and strengthen the country’s position in the global energy transition. The work done by the Bengaluru scientists contributes to this national effort by providing a method that is adaptable, scalable and environmentally safer.
The findings of the team were published in the Advanced Energy Materials journal, marking recognition from the global scientific community and lending credibility to India’s growing contributions to research into advanced materials. Their method not only addresses one of the key hurdles in zinc-ion batteries but offers a foundation for future improvements.
Expanding the scope of innovation
What makes this development particularly significant is its possible applicability beyond a single material, if the activation technique improves performance in other cathode materials as well, wider progress might be achieved in the field of eco-friendly batteries. Researchers working on manganese-based, nickel-based or organic cathode materials may apply similar methods to their structural limitations.
This cross-applicability strengthens the relevance of the work being done by the Bengaluru team and aligns with global efforts toward diversification of energy storage technologies. As the world looks for other alternatives to lithium, innovations that improve reliability and sustainability will increasingly become key.
The research conducted by the CeNS team represents a meaningful step toward advancing zinc-ion batteries, a technology offering safety, sustainability and affordability. By applying a simple activation technique, the scientists proved how structural modification of a well-known material is able to elevate its performance substantially. Their work brings a zinc-based battery closer to practical use and supports the broader goal of transitioning to cleaner and more dependable energy solutions.
















Comments