Scientists have always been drawn to gold not as the shiny allure found in jewellery, but its unique nanoscale properties. In cutting-edge labs, this oldest metal known to humankind has become a key player in shaping futuristic medical technologies smarter biosensors, reliable drug delivery, better diagnostics. However, as a puzzle with movable pieces, a recurring issue was how do gold nanoparticles react when exposed to common molecules such as Amino Acids & Salts and is that behaviour controllable for real-world application?
A Scientific Odyssey Begins
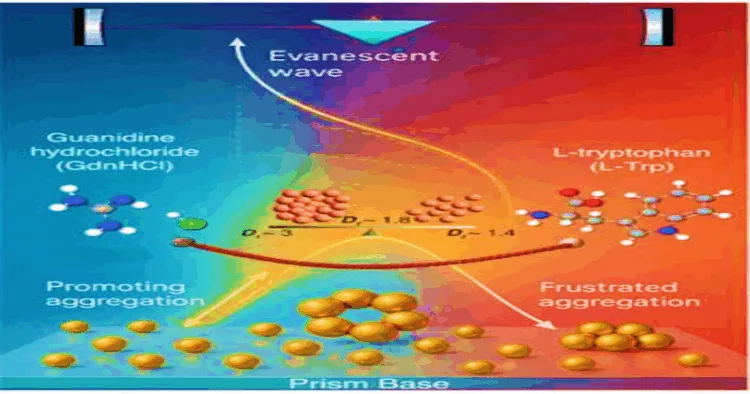
At the S N Bose National Centre for Basic Sciences, a group of diligent researchers embarked on this challenge with curiosity coupled with a passion to make an impactful difference. At the center of their journey were two known but quite different molecules: Guanidine Hydrochloride (GdnHCl), a powerful salt that has earned a place in research labs for its capability to unwind proteins and L-Tryptophan (L-Trp), a fundamental amino acid used in regular diets and commonly linked with sensations of relaxation and sleep.
Guided by Professor Manik Pradhan, the team wondered could these molecules, so deeply woven into both science and life, can alter the fate of gold nanoparticles in aggregation? To find out, they designed a series of experiments that would reveal, for the first time the intricate dance between these molecules and the shimmering gold particles at the heart of frontier technologies.Scientists-have-uncovered-how-gold-nanoparticles.docx
Into the World of Nanoparticles
Gold nanoparticles aren’t just beautiful and they’re remarkable because they interact with light in ways that change depending on their state. They gleam with distinct colours, clustered, their hues and optical signals shift even more dramatically. It’s this unique property that makes them invaluable in biosensors, medical imaging and many more applications. But therein lies a problem, if nanoparticles clump together uncontrollably, the devices built from them can become unreliable.
For years, scientists tried to decode and control this unpredictable clustering, hoping that by controlling it, they could open the door to stable and practical applications. With their new project, the Bose Centre team dared to dig even deeper could they not only control aggregation, but actually sculpt it into new, useful forms?
Molecules in Action
Scientists found with the addition of GdnHCl, the gold nanoparticles lost their natural repulsion and rushed together, forming dense and almost stubbornly tight clusters. For the scientists observing through their sophisticated instruments, it was like watching a crowd of dancers pressed too closely in a ballroom beautiful, but chaotic, the signals jumbling together.
Then came L-Trp. Introduced alongside the powerful salt, the amino acid changed everything. Instead of tight-packed clumps, the nanoparticles began to self-organize in looser, branching structures like dancers weaving into spacious, interconnected patterns. The aggregation wasn’t stopped, but transformed and the researchers called it “frustrated aggregation,” as if the nanoparticle in mid-cluster couldn’t quite complete their embrace because L-Trp kept gently pushing them apart.
Rather than seeing this as a barrier, the research team recognized its promise. Frustrated aggregation hinted at new possibilities: biosensors with more reliable signals, drug delivery systems where particles release their cargo gently and predictably, medical diagnostic tools that function smoothly even when exposed to complex biological mixtures.
Sensing the Invisible: EW-CRDS
To understand what was happening, the team didn’t rely on ordinary tools, they turned to Evanescent Wave Cavity Ringdown Spectroscopy (EW-CRDS), a technique so sensitive it can track minute events on surfaces in real time. Picture a method where evanescent waves faint, ghostly ripples of light not only observe but also probe the shifting arrangements of molecules.
Using EW-CRDS, the scientists watched as L-Trp subtly stabilized the guanidinium ions from GdnHCl, slowing down the rush to aggregate and letting gold nanoparticles form open networks instead of compact knots. This wasn’t just tinkering at the surface it was real-time monitoring of a molecular ballet, one that could be tuned for future technologies.
Collaboration and Wonder
Behind the scientific breakthrough stood a dedicated team of Soumyadipta Chakraborty, Dr. Jayeta Banerjee, Indrayani Patra, Dr. Puspendu Barik and Professor Manik Pradhan.
Their discovery wasn’t just about molecules, light or nanoparticles it was about working together, blending individual expertise, perseverance and creative thinking. The insights were published in the prestigious journal Analytical Chemistry, showcasing the contribution to both fundamental nanoscience and practical innovation.
This study has opened new opportunities in understanding and manipulating nanoparticle behaviour, offering hope for smarter biosensors and more dependable medical technologies. By revealing how typical molecules like GdnHCl and L-Trp shape the aggregation process, the team turned a scientific obstacle into an opportunity.
The tale of gold nanoparticles at the Bose Centre is not simply one of finding an answer, it’s one of the visions of curiosity of man working together and the potential of science to shape common molecules into the blocks of a healthier tomorrow.


















Comments